GAMUNEX-C is celebrating 20 years of helping patients!
GAMUNEX-C continues to be a trusted choice for patients with PIDD, ITP, and CIDP. Thank you for continuing to be a part of the journey with GAMUNEX-C!
Peer-to-Peer Discussions
Join us for our upcoming live and virtual events.
Gamunex Connexions
Take advantage of resources for your office and find support and financial assistance for your patients.
Clinical Resources
Access publications, resources, and links to organizations for the latest insights.

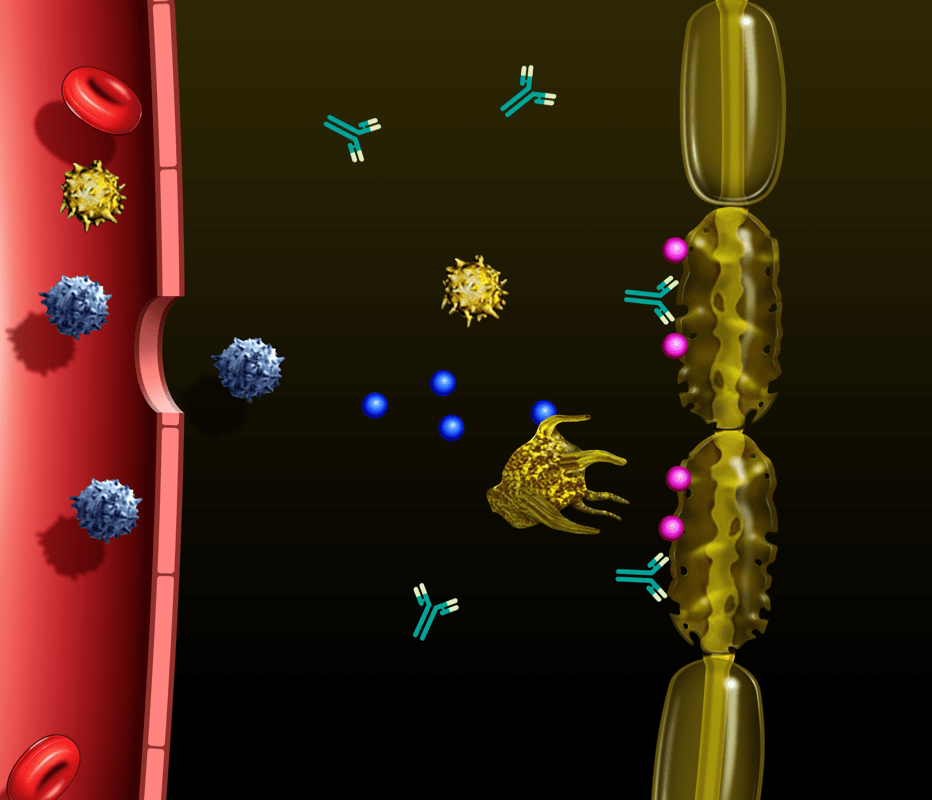
IVIG disrupts inflammation in CIDP through multiple actions2,3
Trusted neuroprotection from inflammation in CIDP with >87% of responders relapse free in the extension phase of the ICE study4-6
Severe hypersensitivity reactions may occur with IVIG products, including GAMUNEX-C. In case of hypersensitivity, discontinue GAMUNEX-C infusion immediately and institute appropriate treatment.

Unique caprylate/chromatography purification yields maximum purity of IgG7-9
Receive updates and important information on GAMUNEX-C
Voluntary withdrawals of GAMUNEX-C 10% lots
Important Safety Information
GAMUNEX®-C (immune globulin injection [human], 10% caprylate/chromatography purified) is indicated for the treatment of primary humoral immunodeficiency disease (PIDD) in patients 2 years of age and older, idiopathic thrombocytopenic purpura (ITP) in adults and children, and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults.
Thrombosis may occur with immune globulin products, including GAMUNEX-C. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. For patients at risk of thrombosis, administer GAMUNEX-C at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with immune globulin intravenous (IVIG) products in predisposed patients. Patients predisposed to renal dysfunction include those with any degree of preexisting renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IVIG products containing sucrose. GAMUNEX-C does not contain sucrose. For patients at risk of renal dysfunction or failure, administer GAMUNEX-C at the minimum concentration available and the minimum infusion rate practicable.
GAMUNEX-C is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Severe hypersensitivity reactions may occur with IVIG products, including GAMUNEX-C. In case of hypersensitivity, discontinue GAMUNEX-C infusion immediately and institute appropriate treatment.
Monitor renal function, including blood urea nitrogen (BUN), serum creatinine, and urine output in patients at risk of developing acute renal failure.
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur in patients receiving IVIG treatment, including GAMUNEX-C.
There have been reports of aseptic meningitis, hemolytic anemia, and noncardiogenic pulmonary edema (transfusion-related acute lung injury [TRALI]) in patients administered with IVIG, including GAMUNEX-C.
The high-dose regimen (1g/kg x 1-2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern.
Because GAMUNEX-C is made from human blood, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Do not administer GAMUNEX-C subcutaneously in patients with ITP because of the risk of hematoma formation.
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of BUN and serum creatinine, before the initial infusion of GAMUNEX-C and at appropriate intervals thereafter.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies, because of the potentially increased risk of thrombosis.
If signs and/or symptoms of hemolysis are present after an infusion of GAMUNEX-C, perform appropriate laboratory testing for confirmation.
If TRALI is suspected, perform appropriate tests for the presence of antineutrophil antibodies and anti-HLA antibodies in both the product and patient's serum.
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield positive serological testing results, with the potential for misleading interpretation.
In clinical studies, the most common adverse reactions with GAMUNEX-C were headache, pyrexia, hypertension, chills, rash, nausea, arthralgia, and asthenia (in CIDP); cough, rhinitis, pharyngitis, headache, asthma, nausea, fever, diarrhea, and sinusitis with intravenous use (in PIDD) and local infusion-site reactions, fatigue, headache, upper respiratory tract infection, arthralgia, diarrhea, nausea, sinusitis, bronchitis, depression, allergic dermatitis, migraine, myalgia, viral infection, and pyrexia with subcutaneous use (in PIDD); and headache, ecchymosis, vomiting, fever, nausea, rash, abdominal pain, back pain, and dyspepsia (in ITP).
The most serious adverse reactions in clinical studies were pulmonary embolism (PE) in 1 subject with a history of PE (in CIDP), an exacerbation of autoimmune pure red cell aplasia in 1 subject (in PIDD), and myocarditis in 1 subject that occurred 50 days post-study drug infusion and was not considered drug related (in ITP).
Please see accompanying full Prescribing Information for GAMUNEX-C.
Terms to know
IG, immune globulin; CIDP, chronic inflammatory demyelinating polyneuropathy; PIDD, primary immunodeficiency disease; ITP, idiopathic thrombocytopenic purpura; Sub Q, subcutaneous; IV, intravenous; ICE, 10% caprylate-chromatography purified immune globulin intravenous (IGIV-C) CIDP efficacy.
References
- Data on file, Grifols.
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7(9):507-517.
- Ritter C, Bobylev I, Lehmann HC. Chronic inflammatory demyelinating polyneuropathy (CIDP): change of serum IgG dimer levels during treatment with intravenous immunoglobulins. J Neuroinflammation. 2015;12:148.
- Khoo A, Frasca J, Schultz D. Measuring disease activity and predicting response to intravenous immunoglobulin in chronic inflammatory demyelinating polyneuropathy. Biomark Res. 2019;7:3.
- Dalakas MC. Potential biomarkers for monitoring therapeutic response in patients with CIDP. J Peripher Nerv Syst. 2011;16(suppl 1):63-67.
- Hughes RAC, Donofrio P, Bril V, et al; on behalf of the ICE Study Group. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136-144.
- Alonso W, Vandeberg P, Lang J, et al. Immune globulin subcutaneous, human 20% solution. Biologicals. 2020;64:34-40.
- GAMUNEX®-C (immune globulin injection [human], 10% caprylate/chromatography purified) Prescribing Information. Grifols.
- Lebing W, Remington KM, Schreiner C, Paul HI. Properties of a new intravenous immunoglobulin (IGIV-C, 10%) produced by virus inactivation with caprylate and column chromatography. Vox Sang. 2003;84(3):193-201.